A 12 year old, female, DSH cat was presented for yearly health check at the local vet.
The cat lived outdoor, was regularly vaccinated, dewormed and (according to the owner) healthy.
Physical examination was unremarkable.
Haematology and biochemistry without clinically significant changes.
The performed blood smear is shown below.
1. How do you interpret the smear?
2. What is your diagnosis?
3. Do you know the pathophysiology and possible reasons behind this change?
4. Do you think the change is diagnostically relevant?
5. What is your suggestion to the owner?
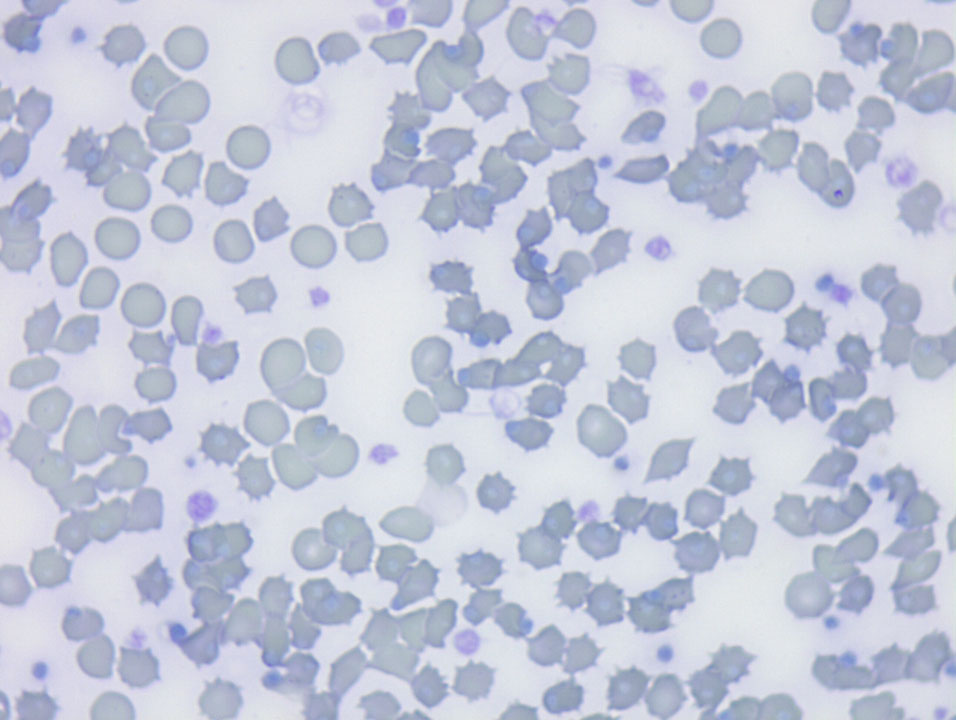
Blood smear, 100x Oli, mod. Romanowsky stain
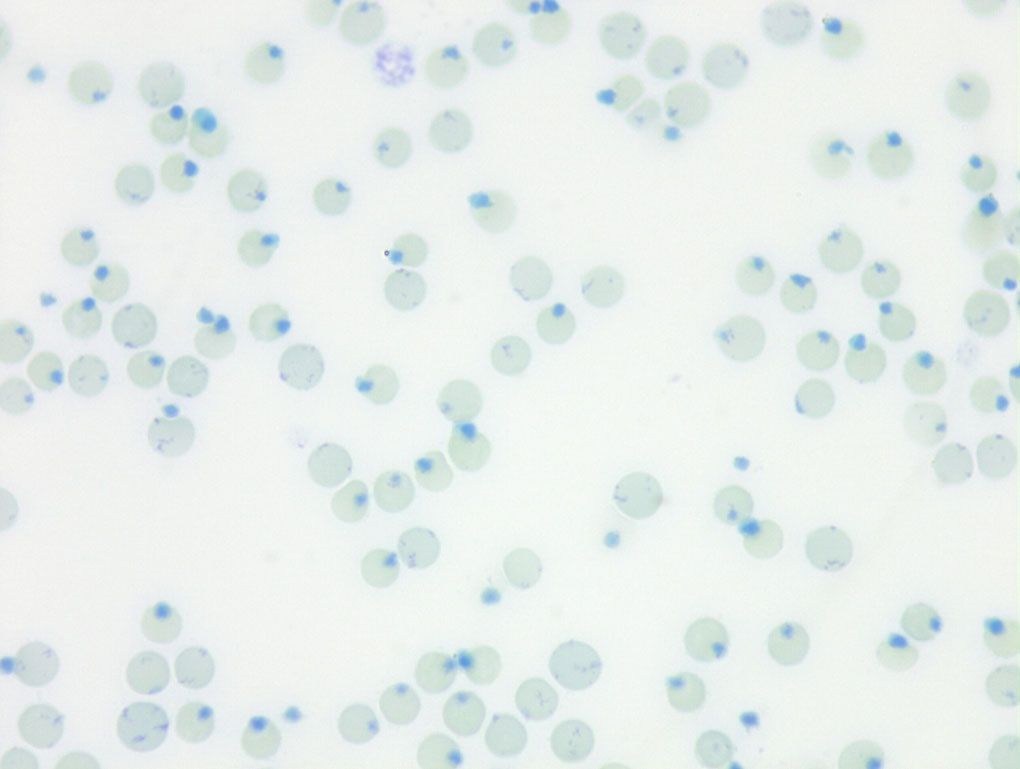
Blood smear, 100x Oli, methylen Blue stain
Answers to the Case
1. How do you interpret the smear?
RBC: high quality blood smear with high number of acantocytes and Heinz bodies (86% of RBC)
WBC: Not assessable
PLT: number unremarkable, high number of macrothrombocytes and reactive PLT
2. What is your diagnosis?
- Acantocytosis
- Heinz body formation
3. Do you know the pathophysiology and possible reasons behind this change?
Acanthocytes
Acanthocytes (spur cells) are RBCs with irregularly spaced, variably sized spicules. They can form when RBC membranes contain excess cholesterol compared to phospholipids. If cholesterol and phospholipids are increased to a similar degree, codocyte formation is more likely than acanthocyte formation (Cooper et al., 1972). Alterations in RBC membrane lipids can result from increased blood cholesterol content (Cooper et al., 1980) or the presence of abnormal plasma lipoprotein composition (Ulibarrena et al., 1994). Another possible contributing factor is the defective repair (acylation of lysophospholipids) of oxidant-damaged RBC phospholipids reported in human patients with cirrhosis and acanthocytosis (Allen and Manning, 1994). Acanthocytes have been recognized in animals with liver disease, possibly because of alterations in plasma lipid composition, which can alter RBC lipid composition (Christopher and Lee, 1994; Shull et al., 1978). They have also been reported in dogs with disorders such as hemangiosarcoma and disseminated intravascular coagulation that result in RBC fragmentation (Weiss et al., 1993).
Marked acanthocytosis is reported to occur in young goats (Holman and Drew, 1964) and some young cattle (McGillivray et al., 1985; Sato and Mizuno, 1982). Acanthocytosis of young goats has been attributed to the presence of HbC at this early stage of development (Jain et al., 1980).
Acanthocytosis in blood is associated with a hetero-geneous group of inherited neurodegenerative disorders in humans (neuroacanthocytosis), resulting in defects of at least four different
proteins (Stevenson and Hardie, 2001). Deficient proteins appear to be important in membranes of neural or muscular tissues in addition to RBCs.
Heinz Body formation
Oxidation of exposed sulfhydryl groups on hemoglobin causes formation of disulfide bonds and distortion of the tertiary structure of the hemoglobin molecule. The result is precipitation of hemoglobin, which may then coalesce to form intracellular inclusions called Heinz bodies. Small, non-pathologic “endogenous” Heinz bodies can be found in blood of many healthy cats, but their presence is abnormal in other species. Increased numbers of these “endogenous” Heinz bodies are found in cats with diabetes mellitus (associated with ketone production), lymphoma and hyperthyroidism. Although many of these cats are not anemic, their red blood cell lifespans are reduced and they have lower hematocrits than cats with the same disease but lower numbers of Heinz bodies.
Heinz bodies may be seen in Wright’s stained smears, especially if they are large and distort the red cell membrane. In contrast, small “endogenous” Heinz bodies, such as those that can be seen in cats, are harder to visualize. Heinz bodies can be easily observed with supravital stains, such as new methylene blue, as illustrated in the picture below.
Extensive Heinz body formation and attachment to the membrane increase the rigidity of the red blood cells and render them susceptible to fragmentation and entrapment in the spleen where they are phagocytized.
Causes of oxidant-induced hemolytic anemia are:
Inherited disorders: Enzyme deficiencies (necessary for enzyme function) in pathways that guard against oxidant injury can result in oxidant-induced hemolytic anemia, e.g. Glucose-6-phosphate dehydrogenase (G6PD) in horses, or methemoglobinemia, e.g. flavin adenine dinucleotide (FAD) deficiency in horses.
Toxins: Wilted red maple leaves (horses, camelids), Pistachia species (horses), Brassica spp. (kale, turnips; cattle), zinc (dogs ingesting pennies minted after 1982), copper (sheep), skunk musk (dogs, red panda), naphthalene (dogs), nitrate (cattle), onions.
Drugs: Acetaminophen, vitamin K1, phenothiazine drenches, benzocaine, propofol. These act as oxidants.
Mineral deficiency: Selenium is necessary for the function of enzymes that protect against oxidant injury. Selenium deficiency in cattle can result in oxidant injury to red blood cells.
4. Do you think the change is diagnostically relevant?
The cat had no clinical signs and messured hematology was unremarkable. Therefore the changes in the blood smear are an incidental finding and are not diagnosticalle relevant.
5. What is your suggestion to the owner?
It was recommended to re-Check hematology about 4 weeks later to rule out developing anemia.
4 weeks later the blood work was unchanged, but the owner reported to the private vet from PU/PD.
The checked fructosamine have strongly increased. DM was hypothized.
Thank you very much for your time and congratulations to your learning success! 👍🏻🧑🏻🎓👨🏻🎓
Please feel free to contact me for further questions!
